Tungsten takes on hydrogen
April 1, 2012
The low solubility of hydrogen in tungsten has led to its use as a gasket material to seal hydrogen in diamond anvil cell experiments at high pressures. This story, reported in J. Phys.: Condens. Matter, begins with a finding by Tim Strobel of the Carnegie Institution of Washington (CIW) that the gasket is not inert. He noticed that a tungsten hydride of WH composition can be formed under pressure. A similar finding was reported by a group at the University of Hyogo earlier.
The Cornell theory group, consisting of both chemists and physicists, and with a history of collaboration with the CIW experimentalists, was intrigued. They asked: Can other WHn phases be stabilized under pressure? And what might they look like?
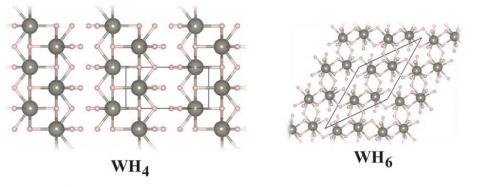
An exploration of the rich structural landscape of tungsten hydrides ensued. Four tungsten-hydrogen compositions—WH, WH2, WH4 and WH6—appear stable, each in its own pressure range. The structures of the predicted extended hydrides are fascinating. They begin with an anti-NiAs-type structure for WH (in agreement with experiment)—a trigonal prism of hydrogens around each tungsten. With an increasing number of Hs in the chemical formula, the number of hydrogens around each tungsten atom increases, while the tungsten atoms remain about the same distance apart.
The theoretical findings led the CIW group to direct efforts towards detecting the higher hydrides. Using excess hydrogen, different forms of tungsten, and heating, resulted in a better characterization of WH (see figure below), but no sign of the predicted higher hydrides. Attempts have been made to explain the absence of the higher hydrides through kinetic and entropy considerations, but essentially a mystery remains, an apparent disagreement between theory and experiment. The story will not end here, and there is at least a caution here for experimentalists: Whatever you use for the gasket, chemistry has a way of rearing its head, especially at high pressure.
This study can be found at: P. Zaleski-Ejgierd et al., J. Phys.: Condens. Matter 24, 155701 (2012).

