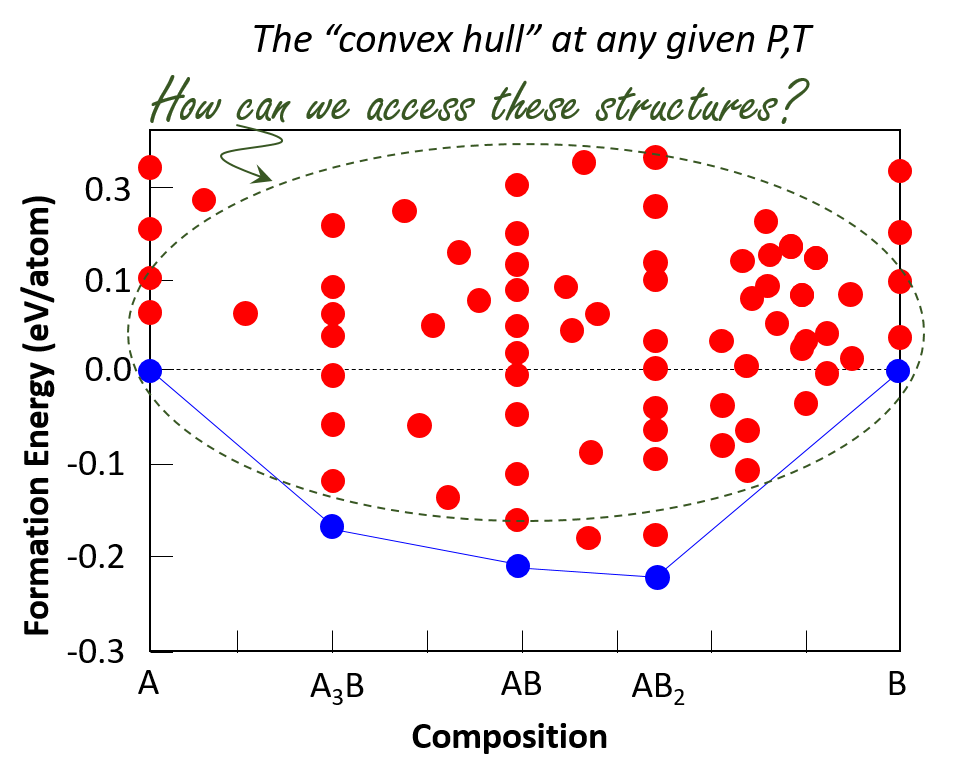
For any given pressure, temperature and composition, we can now predict and synthesize ground-state materials –those that lie on the convex hull– with remarkable success. However, numerous polytypes are predicted to exist above the convex hull, sometimes within only a few kJ/mol. In many cases, there are hundreds or thousands of “energetically plausible” and dynamically stable structures. Given the vast number of potential metastable structures compared with the singular ground state, it is likely that the material with the “best” property possible for a particular application is not the ground state. To what extent are metastable structures accessible? What criteria define accessibility and are there fundamental limits on synthesizability? Are there rational design principles and experimental methods to access these materials?
Of all physical variables, pressure possesses one of the greatest ranges – over sixty orders of magnitude. Pressure is thus a phenomenal tool to characterize fundamental interactions / processes and to gain access to new materials. As pressure is increased, the free energy of the system also increases through contributions to the pV term. At pressures approaching one million times atmospheric pressure, this contribution becomes comparable to the energy of a covalent bond and remarkable atomic arrangements become stabilized.
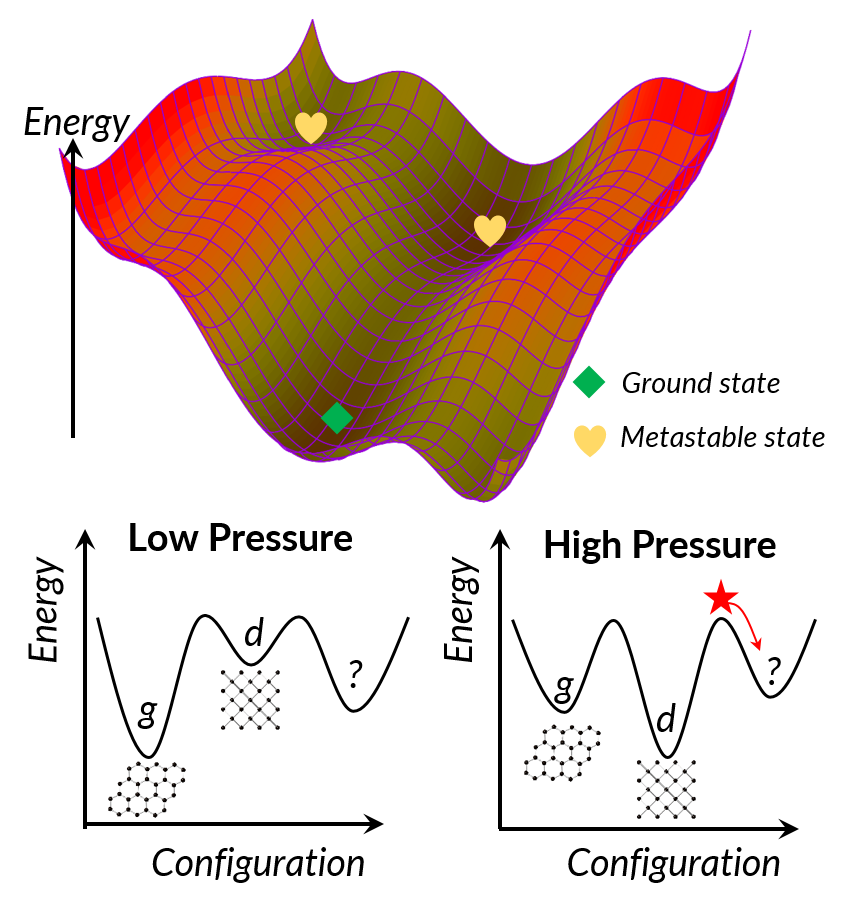
While, the true energy-configurational landscape exists in high-dimensional (3N) space, it can be visualized in three dimensions by reducing the overall dimensionality. The global energy minimum represents the thermodynamic ground state, but many higher-energy local minima may be stabilized by the system's inability to overcome kinetic barriers. Take carbon for example. At low pressure, the lowest-energy structure is graphite, while diamond is the lowest-energy structure at high pressure. Once formed under high-pressure, high-temperature conditions, diamonds may be metastably recovered to ambient conditions, with robust kinetic persistence. Many new materials become thermodynamically stabilized under high-pressure conditions and can be recovered to ambient conditions. New oxides, nitrides, carbides –virtually every class of compounds– are waiting to be discovered.