News
Bhadram wins best poster @GRS
Free Postdoc Venkat Bhadram was one of three selected for best poster awards at the Gordon Research Seminar, which was held before the Gordon Research Conference in July at the Holderness School in New Hampshire.
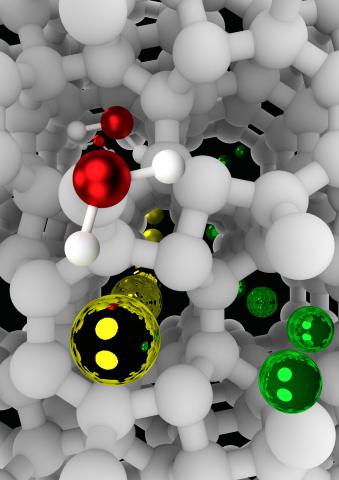
Hydrogen-stuffed, quartz-like water ice
Check out our new paper in JACS.
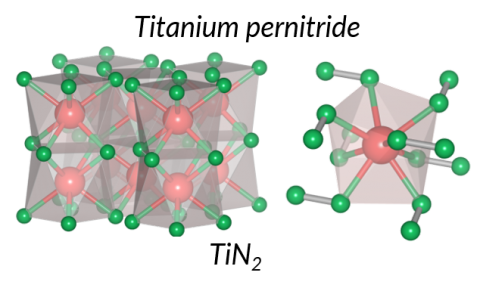
Titanium Pernitride
Scientists from Carnegie have discovered a new transition metal pernitride, TiN2, which is ultraincompressible (bulk modulus ~360-385 GPa), and potentially a superhard material.
Super Silicon in "New Scientist"
The November issue of New Scientist talks about hacking silicon's structure to make it more efficient for use in computer chips and solar panels. The element may have a whole valley named after it, but the atomic structure limits its ability to conduct electricity.
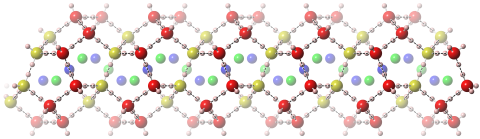
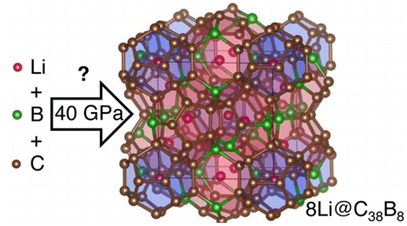
Li-stuffed, B-doped carbon clathrates
The experimental synthesis of carbon clathrate materials is an, as of yet, unaccomplished experimental feat. Inside the cages of these hypothetical compounds there is precious little room, even for the smallest of atoms.
New Allotrope of Silicon
Silicon is the second most-abundant element in the earth's crust. When purified, it takes on a diamond structure, which is essential to modern electronic devices--carbon is to biology as silicon is to technology.
Khattar Selected as 2014 Siemens Competition Semifinalist
The Geophysical Laboratory's Saelig Khattar was named a Semifinalist for the 2014 Siemens Competition in Math, Science & Technology. The Siemens Foundation announced the Semifinalists this week on 22 October 2014.
The Big Squeeze
High-pressure research featured in the NY Times
Check it out here
Supercharged Carbon
A research team from the Geophysical Laboratory, including Oleksandr Kurakevych, Timothy Strobel, Duck Young Kim and George Cody, has reported the synthesis of an ionic semiconductor, Mg2C, under high-pressure, high-temperature conditions, which is fully recoverable to ambient conditio